Is Cf3coo- an Acid or Base
Ch3cook is base What is an acid base neutral. Acid-Base Chemistry 12-1 1.
Solved Classify Each Of The Following Reactants And Products Chegg Com
According to Arrheniuss theory for acid the substance which produces an H ion or proton on dissolving in an aqueous solution is categorized as an acid.

. CH3COOH is Acetic Acid. A Brønsted acid is a proton donor and a Lewis acid is a substance that can share electrons with a Lewis base to form a covalent. This is because a fluorine atom is more electronegative than chlorine.
A Is P R a valid consequence of A1 A2 and A3. Classify each of the following reactants and products as an acid or base according to the Bronsted theory. Chemistry questions and answers.
Its a weak base because its conjugate acid is weak. So for a stronger acid lower cepK_a the negative charge must be more stabilized. Arrhenius theory for acid.
CH3COOH Acetic acid is one of the most common weak acids studied in general chemistry classes. Using the Lewis acidbase definition any molecule that accepts electrons is an acid any molecule that donated electrons in a base. Suppose you are trying to use aqueous base to extract a carboxylic acid from a neutral organic but only 90 of the acid goes into the NaOHwater and 10 stays in the.
So when CH 3 COOH is dissolved. Acetic acid is a simple organic or monocarboxylic acid made up of two carbon two oxygen and four hydrogens with the chemical formula CH3COOH. Consider the acid-base reaction and classify each of the reactants and products as an acid or base according to the Brønsted theory.
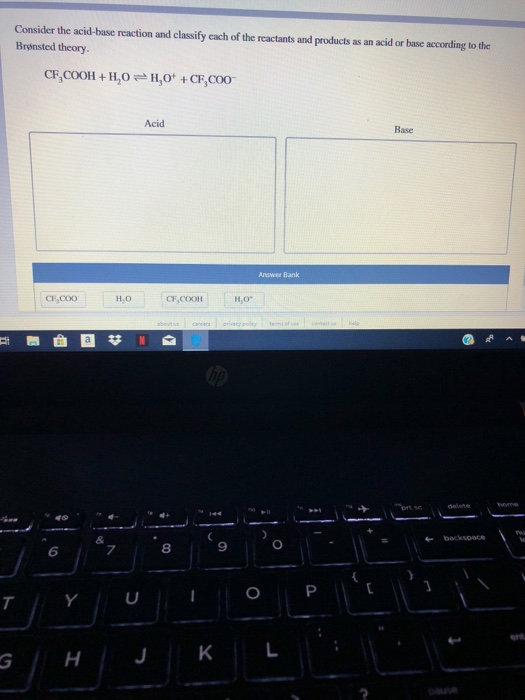
CF3COOH H20 근 H30 CF3COO- Acid Base Answer Bank CECoO CFCOOH 8 9 6. The pairings are Acid. 35 Related Question Answers Found.
A Brønsted-Lowry acid is a proton donor while a Brønsted-Lowry base is a proton acceptor. Very weak means it does not act as a base or acid when you dissolve it in water. Dichloromethane DCM or methylene chloride is an organochloride compound with the formula C H 2 Cl 2.
Classify each product as the conjugate acid or base. Classify each of the following reactants and products as an acid or base according to the Bronsted theory CF3COOH H20 H3O CF3COO-. Terms in this set 100 HCO₃ NH₃ CO₃² NH₄.
Consider the acid-base reaction and classify each of the reactants and products as an acid or base according to the Brønsted theory CF3COOH H20 H30 CF3C00 CF3COOH. Helpful 1Not Helpful 0. Classify the reactants in the following reactions as an acid or base according to the Bronsted-Lowry Definition.
Label each reactant and product in this reaction as a Bronsted acid or base. Acetic acid CH3COOH lewis structure molecular structure hybridization polarity. 3 Conjugate Acids Bases ν Acids react with bases and vice versa ν All acids and bases come with a conjugate paira base or acid respectively that is formed in conjunction with the original species Examples HClaq H 2Ol H 3Oaq Cl-aq acid base conjugate conjugate.
To understand whether CH 3 COOH is an acid or base look out the two important theories of the acid-base concept aArrhenius theory bBronsted-Lowry theory. Try drawing the structure of the conjugate base ceCF_3COO-. Consider the acidbase reaction and classify each of the reactants and products as an acid or base according to the brønsted theory.
It is a weak acid also known as ethanoic acid appears as a colorless liquid and odor like heavy vinegar. For that reason it is helpful to commit CH3COOH along wit. In CCF3COOH the O-H bond will be weakened more than the O-H bond in CCl3COOH.
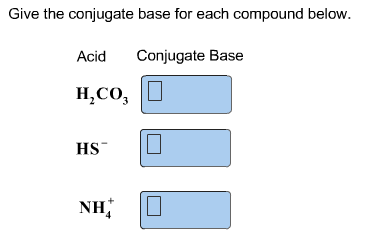
A2 be Q middot R and A3 be Q. Ethylamine is considered a Brønstef-Lowry base because the lone pair of electron on nitrogen atom can form a coordinate bond with a proton to give a positively charged nitrogen atom on ethylamine. CF3COOH H20 H30 CF3COO- Give the conjugate base for each compound below.
In general the more stabilized the negative charge of the conjugate base is the more the equilibrium favors that form thus the more the acid dissociates thus the stronger the acid is. Terms in this set 15 HCO₃ NH₃ CO₃² NH₄. The 3 fluorines in CF3COOH will be able to attract the electrons in the chemical bonds more than the 3 chlorines atoms can.
CF3COOH is a stronger acid than CCl3COOH. FeCl3 is a Lewis acid Cl-. H2CO3 HS-NH4 Let A1 be P Q.
Distinguish between an Arrhenius a Brønsted and a Lewis acid. TFA is a stronger acid than acetic acid having an acid ionisation constant K a that is approximately 34000 times higher as the highly electronegative fluorine atoms and consequent electron-withdrawing nature of the trifluoromethyl group weakens the oxygen-hydrogen bond allowing for greater acidity and stabilises the anionic conjugate baseTFA is widely used in. Classify each product as the conjugate acid or base.
An Arrhenius acid is any compound that contains an H and produces H 1 ions in solution. The conjugate base of CF3COOH is CF3COO- but the conjugate of the other acid is CH3OH methanol and NOT an anion. Answer to A dichloromethane solution contains a base and a neutral compound.
It is made out of Carbon Hydrogen and Oxygen. Trifluoroacetic acid CF3COOH or C2HF3O2 CID 6422 - structure chemical names physical and chemical properties classification patents literature biological. Weak Very weak.
Label each reactant and product in this reaction as a Bronsted acid or base. Answer 1 of 2. Classify the reactants in the following reactions as an acid or base according to the Bronsted-Lowry Definition.
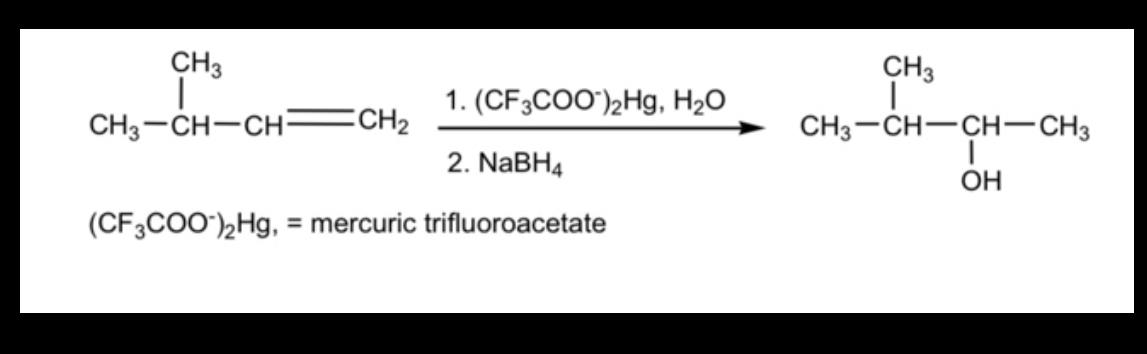
Answered Ch3 Ch3 1 Cf3co0 Hg H2o Ch3 Ch Ch Bartleby

Solved Which Is A Stronger Base Ch3coo Or Cf3coo Cf3coo O Chegg Com

Total Synthesis Stereochemical Assignment And Antimalarial Activity Of Gallinamide A Conroy 2011 Chemistry A European Journal Wiley Online Library
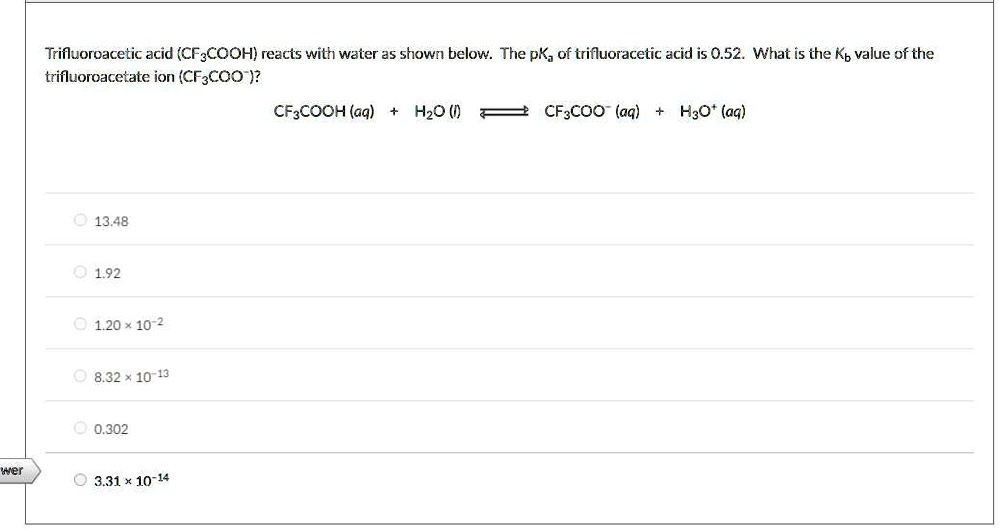
Solved Trifluoroacetic Acid Cf3cooh Reacts With Water A5 Shown Below The Pk Of Trifluoracetic Acid Is 0 52 Whiat Is The Kb Value Of The Trifluoroacetate Ion Cf3coo Cf Cooh Aq Hzo Cf3coo

What Is The Ph Of A 1 5 M Solution Of Trifluoroacetic Acid Cf3co2h With Pka 0 2 Study Com
Solved Consider The Acid Base Reaction And Classify Each Of Chegg Com

13 Question 3 Points Acetic Acid Ch3cooh And Trifluoroacetic Acid Cf3cooh Are Shown By These Particulate Homeworklib

Synthesis Of A Novel Bifunctional Oxyammonium Based Ionic Liquid Application For The Synthesis Of Pyrano 4 3 B Pyrans And Tetrahydrobenzo B Pyrans Zarei 2020 Journal Of The Chinese Chemical Society Wiley Online Library

Photomodulation Of Dna Templated Supramolecular Assemblies Rubio Magnieto 2018 Chemistry A European Journal Wiley Online Library

Making A Case Video Diy Phone Case Phone Accesories Custom Case
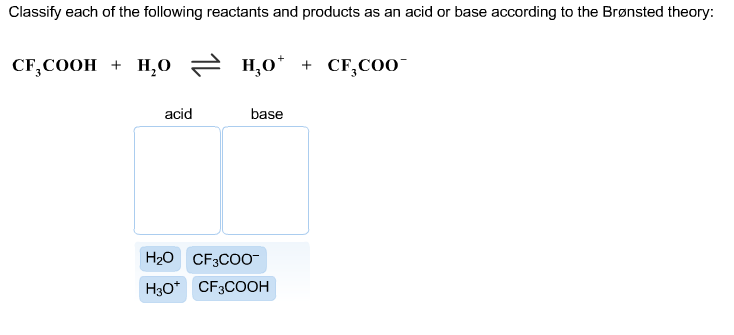
Solved Classify Each Of The Following Reactants And Products Chegg Com
Trifluoroacetic Acid Cf3cooh Pubchem
Nn Dimethyltrifluoroacetamide C4h6f3no Chemspider
Trifluroacetate C2f3o2 Chemspider

Overview On Developed Synthesis Methods Of Triazepane Heterocycles Mohammadi Zeydi 2017 Journal Of The Chinese Chemical Society Wiley Online Library

Oneclass 03 Question 1 Point A See Page 678 Acetic Acid Ch3cooh And Trifluoroacetic Acid Cf3coo

Silver In C Sp2 H Functionalization Liu 2021 Chemcatchem Wiley Online Library

Synthesis Of A Novel Bifunctional Oxyammonium Based Ionic Liquid Application For The Synthesis Of Pyrano 4 3 B Pyrans And Tetrahydrobenzo B Pyrans Zarei 2020 Journal Of The Chinese Chemical Society Wiley Online Library
Comments
Post a Comment